By Jacob Prater
Metals are amazing materials. They can be formed, welded, drawn into wires, and sometimes have their properties changed with heat treatments. Metals don’t rot due to fungi or get eaten up by insects like wood can. And they don’t have issues with UV degradation like many plastics do.
The Basics of Corrosion
But there is one thing that can make these wonderful materials succumb to corrosion. In order to protect metals, one needs to understand a bit about the corrosion process.
While we went through some interesting properties of metals above, the most important one for understanding corrosion is related to electrical properties and chemistry (shiver). Metals are great conductors of electricity as they freely allow electrons to move through them. Related to this property is that metals lose or give up electrons or gain or accept them. The process of a metal losing an electron is the process of oxidation (which confusingly does not require oxygen). The process of a metal gaining an electron is called reduction.
When metals lose or gain electrons, that metal changes its oxidation state. For instance, Iron III (Fe3+) can be reduced to Iron II (Fe2+) and Iron 0 (Fe zero, this one is iron metal). This is part of the process of smelting iron ore to turn it into iron metal and is where we get the term “reduced” or “reduction.”
Way back when humans began smelting iron ore they noticed that there was less material after smelting the ore and thus it was reduced (in weight). Most of what was lost was oxygen and hydrogen, which readily react with Iron III (Fe3+) producing our favorite: rust (more or less, although there a whole host of different types of iron oxide and oxyhydroxide minerals that could be formed).

Two things can be picked up from this little bit of knowledge. The first is that it takes energy to make iron metal, and the second is that iron is more stable under natural conditions in the Iron III (Fe3+) state or as an oxide. Thus, all iron and iron steels will eventually rust, and rust is a lower energy state for iron than as the iron metal. This is why steel always needs some kind of protection from the elements if they are to last in them.
Some corrosion occurs a little differently though, with the same underlying processes occurring in something many of us use often: batteries.
Galvanic action is an interesting beast that leads to corrosion — but also batteries. Way back in 1799 Alessandro Volta invented his voltaic pile, which was in essence the first battery. This battery consisted of a stack of alternating thin wafers of zinc and copper with spacers in between (I think paper was used as it removed direct contact but could absorb the acid easily) all soaked in a brine or sulfuric acid. The battery worked through the action of oxidation and reduction reactions. In this case, the zinc metal was oxidized and the hydrogen in the sulfuric acid was reduced and formed hydrogen gas. In this battery the copper was unscathed but the zinc would slowly be eaten away. Copper was the positive end (cathode) of the battery and zinc the negative (anode).
Galvanization Deters Corrosion
That was a lot of history, so it is time I get to the point. Anodes for batteries can make some really good coatings to stop corrosion. Anodes are oxidizing metals of which zinc is a common one for coating steel in the galvanizing process and thus dramatically reducing the amount of rust on that steel. Whether a particular metal acts as an anode or cathode is dependent on the other metal that it is reacting with and each of their electrochemical properties. How much galvanic action — and thus corrosion — might occur, say between metal panels and fasteners of different metals, is dependent on the electrolyte solution that gets on them. (Always check compatibility! For example, stainless steel and galvanized steel together will speed corrosion.)

Usually this electrolyte is rainwater, but if it is saltwater there is going to be more corrosion from galvanic action as the salt water is a better conductor (of electricity) than the rain water. For this reason (dissolved ions speeding up corrosion), soil contacting (near the soil surface) metals are more likely to corrode than those on a rooftop as the water in the soil contains lots of dissolved ions.
Temperature is a factor in any chemical reaction with the effect of speeding it up, so warmer climate means more rapid corrosion with all other things being equal. Generally speaking, anode (example: zinc) will hold up better in the elements than cathodes (example: iron). Zinc is thus the sacrificial anode in galvanizing steel; it preserves the steel while it (the zinc) slowly corrodes. Other anodes used are magnesium and aluminum. (You have probably noticed that aluminum holds up well against corrosion without any coating on it.) Copper is an interesting case, too, as it makes its own protective, green-colored coating as it oxidizes, preventing further damage to the metal.
Other Ways to Protect Metal
While galvanizing is awesome for corrosion prevention of steel, there are other ways to protect metals. They include but are not limited to, electroplating and anodizing, paints and coatings (including paints that contain anodes), and galvanizing with paint or coatings on top.
The Achilles heel of any coating (including galvanizing) is damage to the coating. This break in the coating can happen anywhere it is bent, stressed, or scratched. Paint or other coatings over the galvanizing can provide an additional barrier and/or a little bit of cushion for impact to reduce stress on the anti-corrosion coating.
This is the beauty of a product like a stone-coated steel shingles. It’s got the durability of steel, they are galvanized, and then they are coated and covered in granules to handle impact. Stone-coated steel is at or near the top end for current metal roof corrosion prevention.
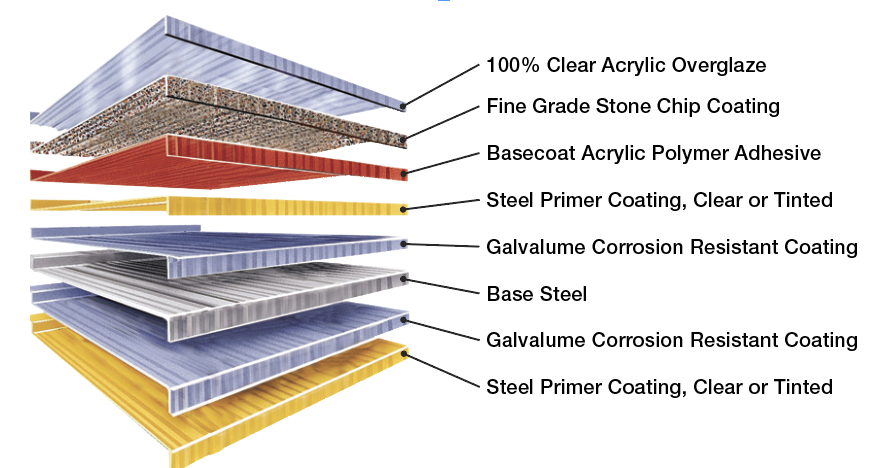
Coatings of epoxy type over galvanized steel are excellent options as well. In that case, you have the epoxy sealing the metal from the elements and galvanizing under that for corrosion protection in case the coating ever is scratched or fails. There are also proprietary coatings that are purported to “self-heal” when scratched. I don’t put that in quotes because they don’t work (they do), but because it is actually chemistry rather than healing.
Coatings in this category often contain something like phosphates to aid in this corrosion prevention. If you have ever used a rust converter on rusted steel, chances are it contained some phosphoric acid and was forming a protective iron phosphate layer where the rust was converted. Some proprietary coatings are even weldable. (Don’t do that with a zinc coating or you can really hurt yourself with fumes; grind off the galvanizing before welding!). And there are lots of products specifically for marine environments (ships, tanks, pipelines) and automotive applications, as well.

There are many options for protecting metals from corrosion and the action of the elements. Your task is to pick the one that fits your customers’ needs and budget. Sometimes that is galvanized steel for barns and grain bins (which are all still standing after many decades) and sometimes that is stone-coated steel shingles for beauty and long-lasting durability. MR
Jacob Prater is a Soil Scientist and Associate Professor in Wisconsin. His passion is natural resource management along with the wise and effective use of those resources to improve human life.