Part I: Metallic Coatings
Metal Roofing Magazine was born as a supplement to Rural Builder magazine in 1999. A few more supplements were published in 2000. In 2001 it was elevated to a stand-alone magazine, and today it is over 20 years old.
This article was originally published in the September 2001 edition of Metal Roofing Magazine. The information is as relevant today as it was two decades ago.

By Derek A. Hodgin, PE, RBEC, CCCA
With so many finish options available for metal roof panels, choosing the right one can be difficult. This article is intended to guide the reader through the selection process by providing basic information on the most popular finishes. Part I is devoted to metallic coatings on steel base sheets; paint systems, coating systems, and unpainted metals will be covered in later articles.
Metals such as copper, zinc, stainless steel, and aluminum form hard and self-regenerative oxide barriers that protect them from rapid corrosion in most circumstances. Although ideal for exposed applications such as roofing, these metals are more expensive and lack the strength of carbon steel. Carbon steel also forms an oxide — rust — but it is soft and permeable, and does not offer guard against further corrosion. For this reason, carbon steel must be protected.
Numerous metallic coatings have been used to protect iron or steel sheets for roofing. Tin and terne, a lead-tin alloy-were common in the 19th century but had to be painted to be effective, as does the modern zinc-tin replacement for terne. Galvanized or zinc-coated steel has been used effectively for more than 160 years. More recently, Galvalume® and similar coatings have joined pure zinc as effective and affordable coatings for steel.
Metal coatings protect the base metal by providing a sacrificial metal (galvanic protection) and/or by creating a physical barrier (barrier protection). A metallic coating may also be painted to provide further protection or to add color to a building. However, unpainted metallic finishes have also gained popularity on steep-slope applications, where the roof provides an architecturally pleasing feature.
Galvanic Protection
Galvanic protection refers to the process by which the base metal acts as the cathode and a sacrificial metal serves as the anode. In the presence of an electrolyte (water), the less noble metal (anode) will be compromised, leaving the more noble metal (cathode) intact1. The intensity of this process is proportional to how far apart the two metals are on the galvanic series (often referred to as the galvanic scale). This series is the simplest way to illustrate the relative electrode potentials of various metals. Zinc is the most common anode due to its relative position on the galvanic series.
A simplified version of the galvanic series is provided (below left) for reference. Roofing contractors are well advised to be familiar with the galvanic series, so as not to introduce conditions that will result in galvanic corrosion.
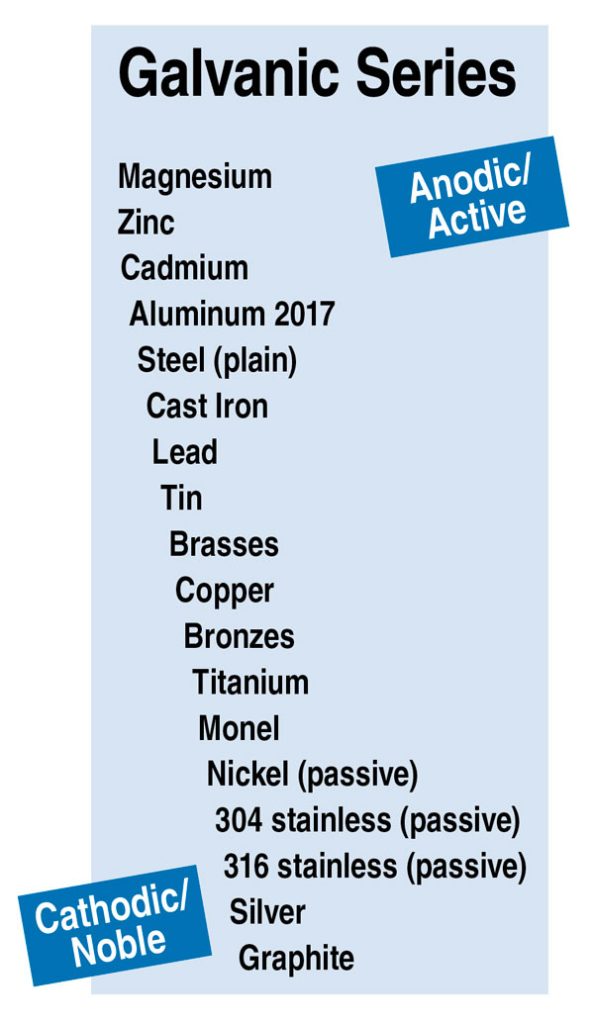
The galvanic series shown includes an approximate grouping of metals based on their anodic and cathodic potential. Again, the farther apart two metals are in the galvanic series, the higher the potential for galvanic corrosion. Roofing contractors should try to use components fabricated from the same metal. If this is not an option, the dissimilar metals should be as close together on the galvanic series as possible. As a last resort, metals with a moderate or significant potential for corrosion should be physically separated by an inert material (i.e. nylon washers or a rubber membrane).
Sacrificial Coatings
The most common sacrificial coating is the galvanized finish, which offers galvanic protection to the noble, cathodic steel by providing a sacrificial anode in the form of zinc. When corrosion occurs, it is the zinc that is compromised, while the steel remains intact. Galvanized finishes are easily identified by the coarseness of their appearance, referred to as spangle.
In general, the thicker the coating, the longer the protection. For all practical purposes, this relationship can be considered as linear1. Depending on the zinc thickness and the severity of the environmental exposure, galvanized roof panels can begin to rust in as little as 10 to 15 years. For this reason, unpainted galvanized panels have typically been discouraged. However, the application of newly developed coatings can greatly enhance the long-term performance of galvanized roof panels, making them a more viable option.
Galvanized coatings have the advantage of being self-healing: The exposed steel will attract zinc from the adjacent coating, healing nicks and providing excellent protection at cut edges and drilled holes. Additionally, galvanized panels are typically less expensive when compared to other protective metal finishes.
The disadvantage of galvanic protection is that the sacrificial metal must always be present. If the sacrificial metal is used up, the base metal will begin to corrode. Additionally, zinc coatings are not resistant to acids and alkalis, and should always be topcoated in chemically aggressive environments.
The minimum zinc coating weights for galvanized steel are designated by ASTM in Specification A924 (formerly A525). Coating weights for galvanized sheets vary, but for roofing applications are typically G90 or 0.90 ounces of zinc per square foot, total both sides. The coating is about 0.76 mils thick per side.


Barrier Coatings
Barriers protect the base metal from corrosion by keeping it free from moisture and oxygen. Paint is the most common barrier. Metal barrier coatings consist of metals such as lead or aluminum that have high resistance to corrosion. While effective, barriers must be maintained. Once a barrier is compromised by weathering, scratches, abrasions, or chemical reactions, the base metal will be vulnerable to corrosion.
Aluminum is the most common metal barrier coating for architectural steel. Aluminized steel was introduced in the 1930s, but was not used in roofing applications until 1954. The application of commercially pure aluminum to steel sheet is a process that was developed by Armco Steel Inc.1 The aluminum coating provides a barrier to the underlying steel base metal. While two types of aluminized steel are available (Type I and Type II), roofing applications are limited to Type II. When exposed to weather, the aluminum coating will form aluminum oxide. Unlike zinc oxide, aluminum oxide is not soluble. For this reason, the durability of aluminized steel is not proportional to the thickness of the aluminum coating.
The minimum aluminum coating weights for aluminized steel are designated by ASTM in Specification A924 (formerly A463). Coating weights for aluminized steel roofing applications are typically 0.65 ounces of aluminum per square foot (designated as T2-65) total both sides. The coating is about 1.1 mils thick per side. This coating carries a limited 20-year warranty against panel perforation due to normal atmospheric corrosion. However, 20-year exposure testing has shown that in most environments it will far outlive and possibly even double the warranted life.
Aluminized steel can be used in painted or unpainted conditions. Its use in roofing has been limited, in part due to the difficulty of protecting cut or drilled edges from corrosion.
Combination Sacrificial/Barrier Coatings
The most common combination coating used in roofing is Galvalume® (marketed as Zincalume® in some areas). Introduced nearly 30 years ago, this zinc-aluminum coating provides both galvanic and barrier protection. The zinc (approximately 45 percent by weight) provides the sacrificial protection, and the aluminum (55 percent by weight) provides the barrier protection. This hybrid finish has become extremely popular for low-slope (less than 3:12) roof applications as well as architectural applications where a “bare-metal” look is desired. In 1995, it was reported that over 5 billion sq. ft. of Galvalume roofing panels had been installed since its introduction3. Due to the combination of protection mechanisms, this finish has the ability to provide a longer service life than a galvanized finish in the same exposure. Current marketing literature suggests a service life of 30 years or more in most environments, without major maintenance.
The minimum coating weights for Galvalume are designated by ASTM in Specification A792. Coating weights for Galvalume roofing applications are typically 0.55 ounces per square foot, total both sides. The coating is about 0.9 mils thick per side.
Basic Considerations
Visibility. The first consideration in choosing a metal coating is whether the roof will be visible from the ground. Designers typically refer to roofs as low-slope or architectural. Low-slope roofs typically cover large one-story commercial buildings and are not visible from the ground. Architectural roofs have steeper slopes (typically 3:12 or more) and are visible from the ground.
Many buildings include both types of roofs. A typical example of this is the modern shopping center (strip mall), which typically consists of a pre-engineered metal building with a low-slope roof. The front and side elevations are often clad with brick or stucco with a steep-slope roof or mansard extending from the front elevation to cover a walkway. Regardless of the application visibility will often determine the type of finish you should consider.
Visibility also relates directly to the roof’s ability to drain: The steeper the slope, the less the metal roofing is exposed to water. For this reason, steeper slope roof panels can be expected to retain their finish longer. As the slope decreases, careful consideration should be given to proper waterproofing details and the durability of the selected finish.
Adverse conditions. Exposure to harmful environments or conditions should also be a top consideration. A few examples2:
1. Contact with strong acids should be avoided. When using aluminum or aluminum alloy, strong alkalis can be detrimental. For this reason, exposure to wet cementitious mortars should be avoided. If necessary, an acrylic coating can sometimes be used to protect the metallic coating from mortar. Highly alkaline cleanser, sometimes used to clean HVAC equipment, should be avoided on rooftops with metallic coatings.
2. Zinc and aluminum are both analytic metals and should be isolated from electrolytic contact with more noble (cathodic) metals such as lead and copper. Flashing should be constructed from the same metal as the roof covering whenever possible.
3. Runoff from copper will act as an electrolyte and cause rapid galvanic corrosion of a metallic coating. Rooftop equipment that includes copper lines that will drip onto the roof should be avoided.
4. Although coastal applications are common, exposure to salt should be limited due to corrosion. For this reason, metallic coatings in coastal environments can be expected to have a somewhat shorter service life. Some manufacturers of metal roof panels will limit or not offer a warranty for projects located at the coast. If a warranty is considered important, this is a topic that should be addressed early in the planning stages of a project.
5. Aluminum and aluminum coatings are sensitive to graphite. In a wet climate, a heavy graphite pencil mark can corrode metallic coatings containing aluminum in as little as two to three years. The process will take slightly longer in drier climates.
Conclusions
Metallic coatings of many varieties have demonstrated years of successful performance on a wide variety of applications. Advances in technology have resulted in a wide selection of metallic coating options for building owners, architects, and roofing contractors. Each successful project should include a conscientious effort to match to the metal roofing components with the desired appearance and function of the building. However, as with most construction projects, proper material selection and attention to detail remain key to long term performance. MR
References
1 Architectural and Low Slope Metal Roofing Systems, Roofing Industry Educational Inst. (RIEI), Feb. 1999.
2 Robert M. Haddock, “Metal Roofing From A (Aluminum) To Z (Zinc),” RSI Magazine, Sept. 1992.
3 Galvalume Sheet Producers of North America, Galvalume Standing Seam Roofs: 20+ Years of Proven Performance, June 1995.
Galvalume® is a trademark of BIEC International Inc.
Derek A. Hodgin has 30 years of experience as an engineering consultant and is responsible for facility condition inspections, failure analysis, damage assessments, and forensic engineering investigations of all types of structures. His experience includes failure analysis of a wide variety of building enclosures and roof systems. He has investigated and testified regarding the performance of various building products including fire retardant treated (FRT) wood, exterior insulation and finish systems (EIFS), hardboard siding and trim. Derek has also designed, permitted and investigated failures of civil and coastal projects such as residential and commercial developments, marinas, docks, shoreline stabilization and retaining systems, basin and channel dredging. A large part of his projects have included analysis of deficient construction cases including roofs, exterior walls, windows, doors, structural framing, civil site work and building code review. He has investigated and testified regarding various types of personal injury cases including slip/trip and fall, railing failures, swing collapse and ladder accidents. Derek has performed engineering assessments of hurricane, tornado, hail, wind, ice and fire-related damages for a wide variety of commercial and residential structures in the United States and Caribbean.